Hydrogen is one of the possible alloying elements in the Earth’s core, but its siderophile (iron-loving) nature is debated.
In a new study by the University of Tokyo, scientists experimentally examined hydrogen partitioning between molten iron and silicate melt at 30–60 gigapascals and 3100–4600 kelvin. The experiment involved a diamond anvil and chemicals in simulating the core of the young Earth.
The outcomes revealed that the hydrogen could bond strongly with iron in extreme conditions. This signifies that many hydrogens in the Earth’s core come from water from bombardments billions of years ago.
To peer deep inside the Earth, scientists used seismic data techniques to discover things like the composition and density of subterranean material. However long these sorts of estimations have been occurring, something that has stood apart is that the core is fundamentally made of iron. Yet, its density, precisely that of the liquid part, is lower than expected.
This prompt scientists to believe that there must be copious light elements along with the iron. Therefore, for the first time, scientists examined the behavior of water in laboratory experiments involving metallic iron and silicate compounds that accurately simulate the metal-silicate (core-mantle) reactions during Earth’s formation.
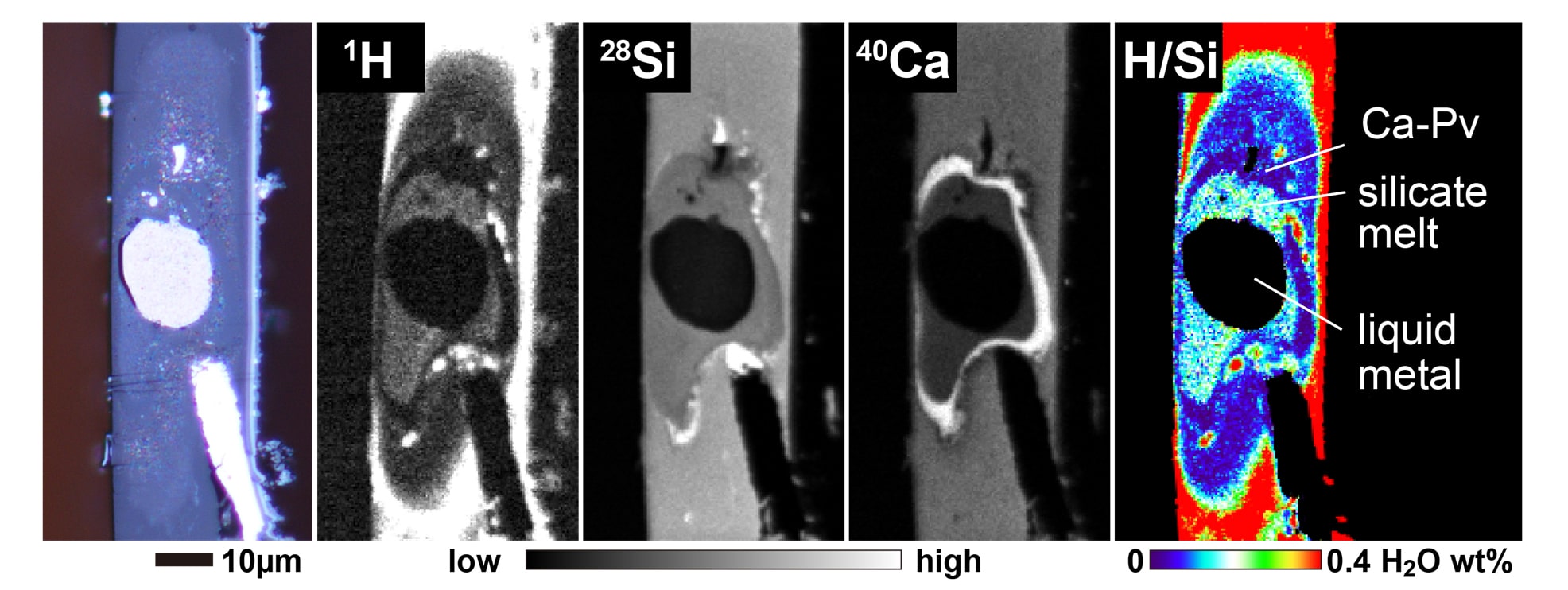
They found that when water meets iron, most of the hydrogen dissolves into the metal while the oxygen reacts with iron and goes into the silicate materials.
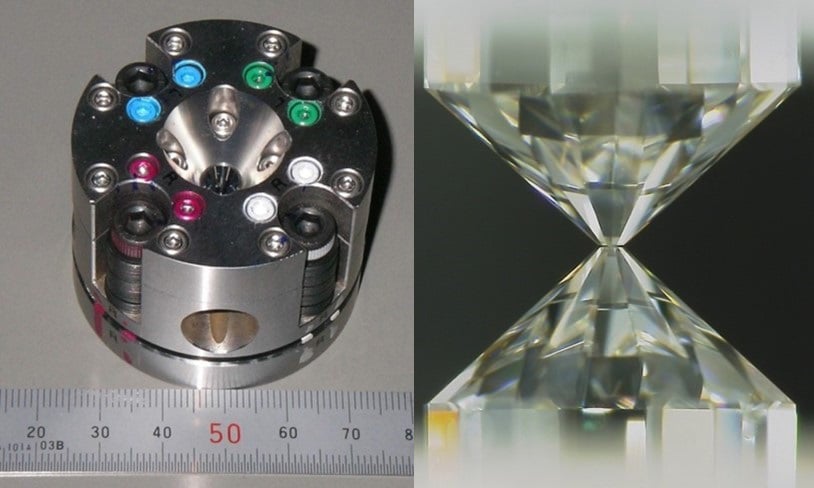
Shoh Tagawa, a Ph.D. student at the Department of Earth and Planetary Science at the University of Tokyo during the study said, “At the temperatures and pressures we are used to on the surface, hydrogen does not bond with iron, but we wondered if it were possible under more extreme conditions. Such extreme temperatures and pressures are not easy to reproduce, and the best way to achieve them in the lab was to use an anvil made of diamond. This can impart pressures of 30-60 gigapascals in temperatures of 3,100-4,600 kelvin. This is a good simulation of the Earth’s core formation.”
Scientists used metal and water-bearing silicate similar to those found in Earth’s core and mantle. They then compressed them in the diamond anvil while simultaneously heating the sample with a laser.
Using secondary ion mass spectroscopy, they observed what was going on in the sample. This allowed them to confirm their hypothesis that hydrogen bonds with iron explain the apparent lack of ocean water. Hydrogen is said to be iron-loving or siderophile.
Hirose said, “This finding allows us to explore something that affects us in quite a profound way. That hydrogen is siderophile under high pressure tells us that much of the water that came to Earth in mass bombardments during its formation might be in the core as hydrogen today. We estimate there might be as much as 70 oceans’ worth of hydrogen locked away down there. Had this remained on the surface as water, the Earth may never have known landlikeand life as we know it would never have evolved.”
Journal Reference:
- Tagawa, S., Sakamoto, N., Hirose, K. et al. Experimental evidence for hydrogen incorporation into Earth’s core. Nat Commun 12, 2588 (2021). DOI: 10.1038/s41467-021-22035-0
Continue reading Hydrogen can bond strongly with iron in extreme conditions on Tech Explorist.
0 comments:
Post a Comment