The nucleation process occurs while forming a crystal from a solution, a liquid, or a vapor, in which an atomic cluster first takes place. Nucleation in atomic crystallization remains poorly understood, despite advances in classical nucleation theory.
According to classical nucleation theory, the process involves a spontaneous transition from disordered to crystalline states, but a detailed understanding of dynamics requires further investigation. The theory suggests that crystals form one atom at a time, steadily increasing the level of order.
Modern studies have also observed a two-step nucleation process, where a temporary, high-energy structure is first formed, which then transforms into a stable crystal.
A new study by the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) suggests that reality is far more complicated than thought.
According to the latest findings, instead of clustering one by one, the atoms self-organize themselves, fall apart, rearrange and reorganize several times before creating a crystal. Scientists observed this rapid process using an advanced electron microscope.
The study offers a detailed view of the early stages of crystal formation.
Peter Marcus, one of the study’s lead authors, said, “As scientists seek to control matter at smaller length scales to produce new materials and devices, this study helps us understand exactly how some crystals form.”
Won Chul Lee, one of the professors guiding the project, describes it this way, explained, “In line with conventional understanding, once the crystals in the study reached a certain size, they no longer returned to the disordered, unstable state.”
“Imagine each atom as a Lego brick, then instead of building a house one brick at a time, it turns out that the bricks repeatedly fit together and break apart again until they are finally strong enough to stay together. However, once the foundation is set, more bricks can be added without disrupting the overall structure.”
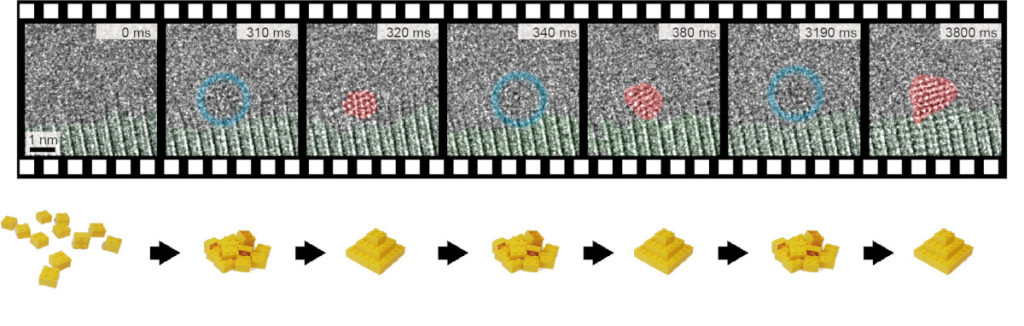
Thanks to newly developed detectors on TEAM I, the team was able to see unstable structures. The TEAM I is one of the world’s most powerful electron microscopes. Using the microscope, scientists could capture real-time, atomic-resolution images at speeds up to 625 frames per second. Scientists also observed individual gold atoms as they formed into crystals, broke apart into individual atoms, and then reformed repeatedly into different crystal configurations before finally stabilizing.
Ercius said, “Slower observations would miss this very fast, reversible process and just see a blur instead of the transitions, which explains why this nucleation behavior has never been seen before.”
The phenomenon occurs because crystal formation is an exothermic process- it releases energy. When atoms attach to the tiny nuclei, the very energy released can raise the local “temperature” and melt the crystal.
In this way, the initial crystal formation process works against itself, fluctuating between order and disorder many times before building a stable enough nucleus to withstand the heat. The research team validated this interpretation of their experimental observations by performing calculations of binding reactions between a hypothetical gold atom and a nanocrystal.
One of the study’s lead authors, Jungwon Park, summarized the work: “From a scientific point of view, we discovered a new principle of crystal nucleation process, and we proved it experimentally.”
Scientists are now looking forward to developing more fast detectors which could be used to image the process at higher speeds. This could help them in understanding if there are more features of nucleation hidden in the atomic chaos.
Journal Reference:
- Sungho Jeon et al. Reversible disorder-order transitions in atomic crystal nucleation. DOI: 10.1126/science.aaz7555
Continue reading Scientists observed the first milliseconds of crystal formation on Tech Explorist.

0 comments:
Post a Comment