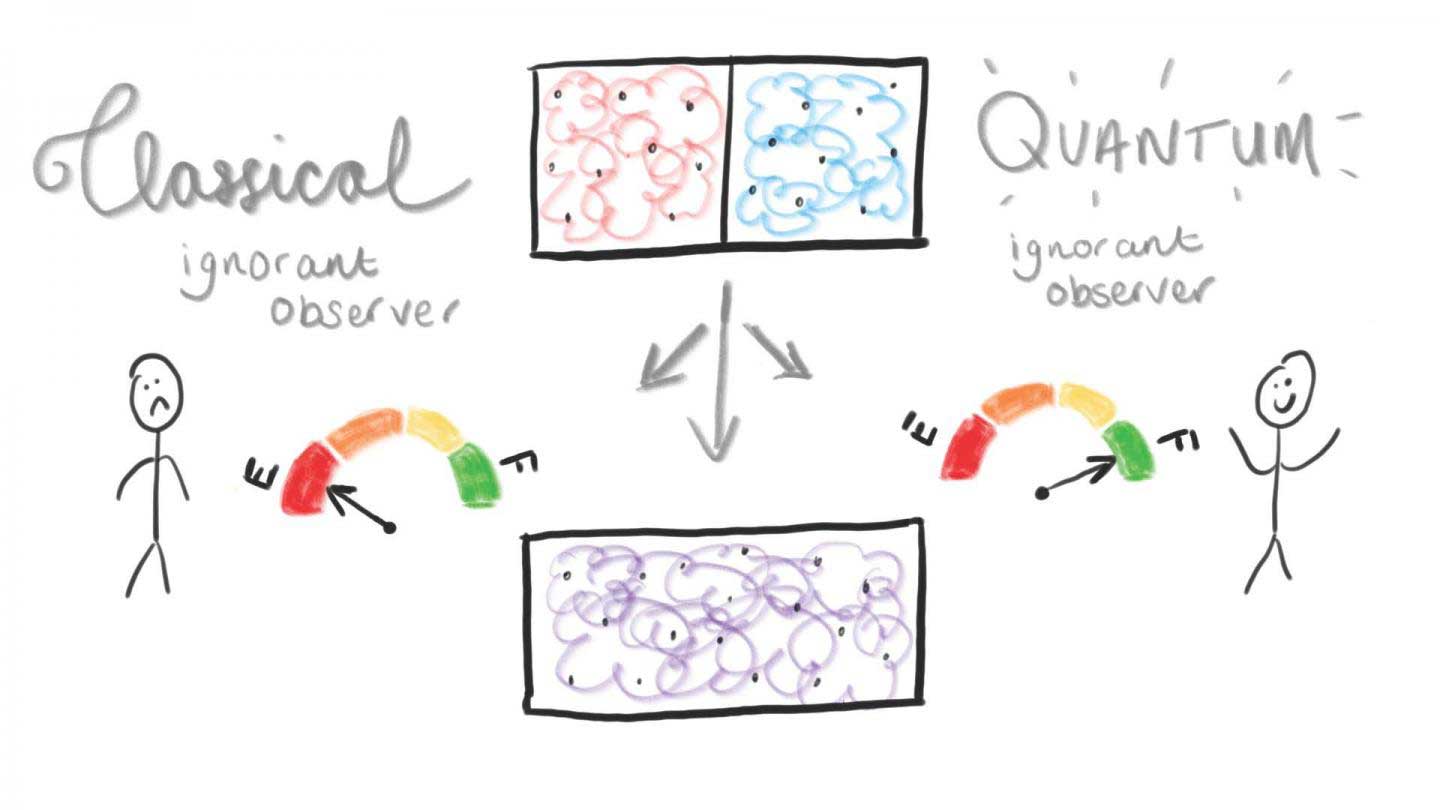
Gibbs paradox considers two gases on either side of a box, separated by a partition and equal volume and pressure. If the gases are identical, then the box is already in thermal equilibrium, and nothing changes after removing the partition. If the gases are distinct, they mix and expand to fill the volume independently, approaching thermal equilibrium with a corresponding entropy increase.
What if the gases differ in some unobservable or negligible way—should we ascribe an entropy increase to the mixing process or not? This question sits uncomfortably with the view that thermodynamical entropy is an objective physical quantity.
Moving the thought experiment into the quantum realm, Mathematicians from the University of Nottingham spotted a significant difference in information and control roles between classical and quantum thermodynamics.
Their new quantum version of the Gibbs paradox experiment could pave the way to develop quantum heat engines.
Scientists developed a theory based on mixing two quantum gases – one red and one blue, otherwise identical – which start separated and then mix in a box. An increase in entropy measures the uniformity of the system.
If the observer then puts on purple-tinted glasses and repeats the process, the gases look the same, so it appears as if nothing changes. In this case, the entropy change is zero.
Benjamin Yadin and Benjamin Morris, lead authors of the study, said, “Our findings seem odd because we expect physical quantities such as entropy to have meaning independent of who calculates them. To resolve the paradox, we must realize that thermodynamics tells us what useful things can be done by an experimenter who has devices with specific capabilities. For example, a heated expanding gas can be used to drive an engine. To extract work (useful energy) from the mixing process, you need a device that can “see” the difference between red and blue gases.”
Considering the circumstance when the system turns out to be significant, where quantum behavior would normally disappear, the scientists found that the quantum ignorant observer can separate as much work as though they had the option to recognize the gases.
Controlling these gases with a large quantum device would behave completely uniquely in contrast to a classical macroscopic heat engine. This phenomenon results from the existence of special superposition states that encode more information than is available classically.
Professor Gerardo Adesso said: “Despite a century of research, there are so many aspects we don’t know, or we don’t understand yet at the heart of quantum mechanics. However, such a fundamental ignorance doesn’t prevent us from putting quantum features to good use, as our work reveals. We hope our theoretical study can inspire exciting developments in quantum thermodynamics’ burgeoning field and catalyze further progress in the ongoing race for quantum-enhanced technologies.”
“Quantum heat engines are microscopic versions of our everyday heaters and refrigerators, which may be realized with just one or a few atoms (as already experimentally verified) and whose performance can be boosted by genuine quantum effects such as superposition and entanglement. Presently, to see our quantum Gibbs paradox played out in a laboratory would require exquisite control over the system parameters, something which may be possible in fine-tuned “optical lattice” systems or Bose-Einstein condensates – we are currently at work to design such proposals in collaboration with experimental groups.”
Journal Reference:
- Yadin et al. Mixing indistinguishable systems leads to a quantum Gibbs paradox. Nat Commun 12, 1471 (2021). DOI: 10.1038/s41467-021-21620-7
Continue reading A new quantum version of a 150-year-old thermodynamical thought experiment on Tech Explorist.
0 comments:
Post a Comment